TMP team – Solubility and Cristallisation phenomena
Partnairs : Christelle GOUTAUDIER (Resp.), Jean-Jacques COUNIOUX, Lobna MANOUBI
The aim of the Solubility and Crystallization Phenomena activity is to study the equilibrium conditions (P, T, x) of the different phases of a thermodynamic system in order to control complex chemical systems. The studies are focused mainly on inorganic solid phases and their precipitation in solution (aqueous or organic medium). They have multiple applications in many synthesis or separation processes involving solid / liquid equilibria: inorganic synthesis, crystal growth, extraction, purification, in close relationship with the industrial environment.
Synthesis of mixed Cobalt/Nickel oxides: control of the composition of solid solutions used as precursors (in collaboration with SETTAT University, Morocco)
Teyssier A. et al., “Partition of metallic cations between aqueous solutions and mixed crystals of hydrated Cobalt and Nickel nitrates: modeling of the influence of temperature and composition”, J. Chem. Thermodynamics 118 (2018) 274-282.
Management of Sodium Borohydride hydrolysis products during hydrogen generation (in collaboration with CEA-Liten, Grenoble)
Stability of NaBH4 aqueous solutions during hydrogen generation by NaBH4 hydrolysis.
Vilarinho-Franco T., PhD thesis, Université Claude Bernard Lyon 1, n°158-2013.
Critical point coordinates of a demixing curve (in collaboration with ProSim society)
Goutaudier C. et al., “Calculation of critical point coordinates of ternary miscibility gap from experimental tie-line.”, Chem. Eng. Res. and Design 92 (2014), 2008-2016.
Clogging of uranium ore treatment plants (in collaboration with AREVA)
Teyssier A. et al., “Contribution to the quaternary system H2O, Al3+, Ca2+ // O2-, SO42- : solid-liquid equilibria in the ternary systems Al2(SO4)3-CaSO4-H2O and Al2O3-SO3-H2O at 25°C.”, Fluid Phase Equilibria 409 (2016), 388-398.
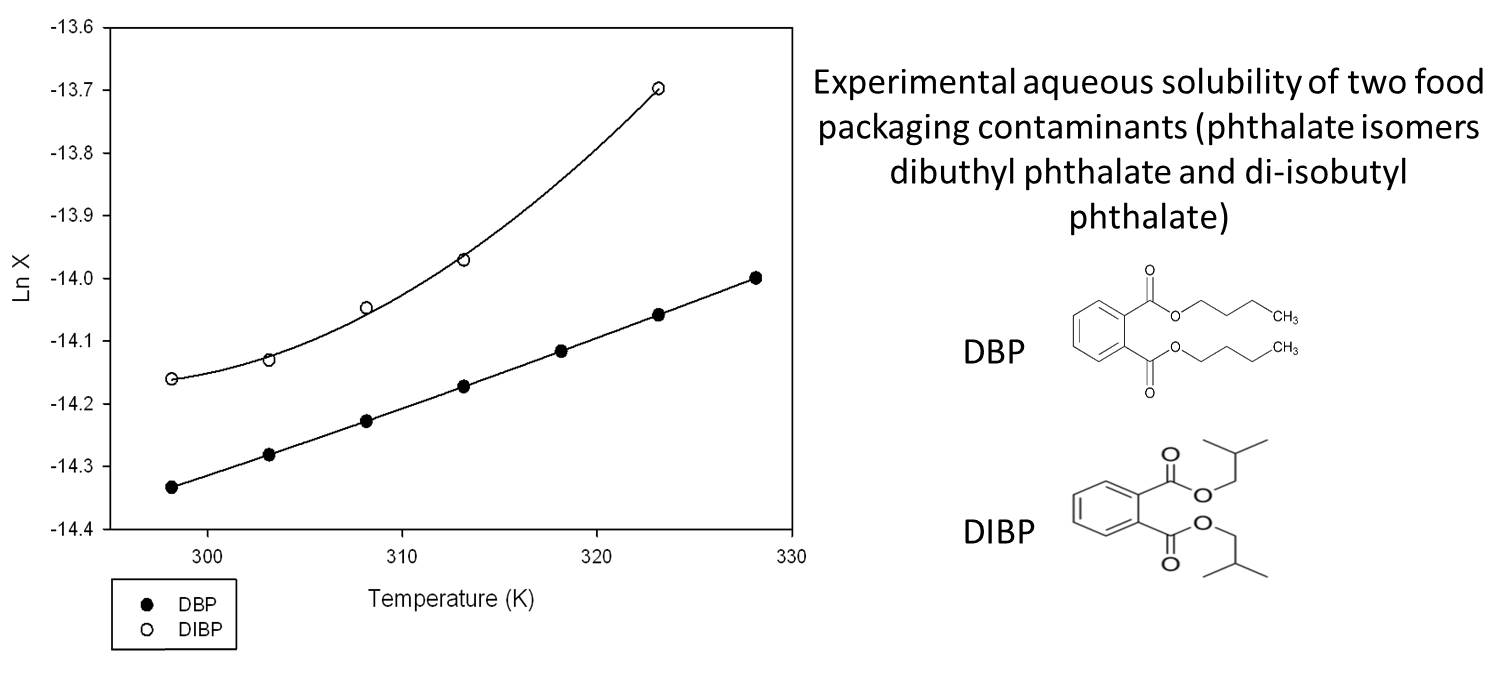
Solubility of hydrophobic organic compounds
Ishak H. et al., “Aqueous solubility, vapor pressure and octanol-water partition coefficient of two phtalate isomers, dibutyl phthalate and di-isobutyl phthalate, contaminants of recycled food packages.”, Fluid Phase Equilibria 427 (2016), 362-370.